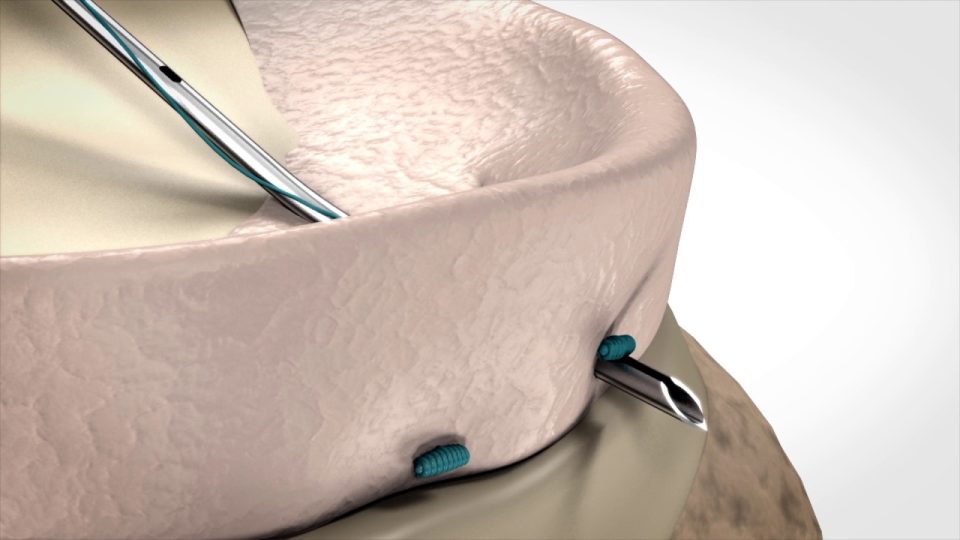
In a significant development for the medical device industry, Arcuro Medical Ltd., a portfolio company of The Trendlines Group (SGX: 42T, OTCQX: TRNLY) has announced that it has received FDA 510(k) clearance for its SuperBall-RC system, designed for use in rotator cuff repair procedures. This clearance marks a crucial milestone for the company, as it aims to address the high re-tear rates associated with current rotator cuff surgeries, which can range from 20% to 40% in patients over 50.
Rotator cuff injuries are common and often require surgical intervention. However, the success of these surgeries is frequently compromised by re-tears, which can occur due to various factors, including the size of the tear, patient age, and surgical technique. Current methods of rotator cuff augmentation using biologic or biosynthetic grafts can help mitigate these risks but are technically demanding and require precise surgical skills.
Arcuro’s SuperBall-RC system is based on the company’s successful SuperBall technology platform, which has been used in over 5,000 meniscus repairs. This new device is designed to facilitate safe and easy fixation of rotator cuff augmentation grafts, offering a promising alternative for surgeons looking to enhance healing outcomes. According to Philip Davidson, MD, Arcuro’s Medical Director, “The SuperBall-RC has exceeded my expectations and offers a very attractive alternative to fixate augmentation patches and enhance healing.”
Jamal Rushdy, Arcuro’s CEO, expressed enthusiasm for the regulatory clearance, praising the company’s product development and regulatory teams for their work. This milestone is expected to improve rotator cuff repair outcomes for patients and marks a significant step forward for the company.
The SuperBall-RC system will initially enter a limited user release in the second quarter of 2025, with a full launch anticipated in the second half of the year. This accelerated timeline is a direct result of the timely FDA clearance.
Arcuro Medical, headquartered in Israel with U.S. operations in Minneapolis and Naples, is expanding its global distribution network to introduce the SuperBall technology to healthcare professionals worldwide.